Review Muscle Tone and Abnormalities in Muscle Tone by Completing Each Sentence Below
Spasticity is a common phenomenon seen in neurologic disorders that outcome in loss of mobility and may produce pain due to musculus spasms. It is a state of sustained increment in tone of a muscle when it is passively lengthened.
Definitions of Spasticity
In simple terms of clinical neurology, spasticity is defined as increased resistance to passive movement due to a lowered threshold of tonic and phasic stretch reflexes (Shush et al., 1972).
Physiologically spasticity is divers as a motor disorder characterized by a velocity dependent increment in the tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflexes as one component of the upper motor neuron (UMN) syndrome (Lance, 1980). The velocity dependent increment in resistance to passive stretch often melts suddenly resulting in clasp-knife phenomenon. The definition of spasticity was further elaborated by addition of several features of spastic paresis to form a more comprehensive moving-picture show of UMN syndrome which are described below (Young, 1989; Delwaide and Gerard, 1993).
1. In a patient with spasticity, brisk tendon jerks sometimes accompanied past clonus and velocity dependent musculus hypertonia to stretch preferentially affecting certain musculus groups, are the furnishings of a combination of hyperexcitability of an afferent pathway to motor neurons and disturbed processing of other peripheral afferent pathways at the spinal cord level.
2. In spasticity, other positive symptoms or signs such every bit flexor (or extensor) spasm, clasp pocketknife phenomenon. Babinski sign, exaggerated cutaneous withdrawal (flexor, pain) reflexes, autonomic hyperflexia, dystonia, and contractures may limit voluntary motion and cause discomfort.
3. In addition to the in a higher place features, several negative features are also included in spastic states such as paresis, lack of dexterity and fatigability.
Mechanism of Spasticity
In the pathophysiology of spasticity and spastic paretic syndrome there are two wide categories of inter-related influencing mechanisms namely:
1. Spinal machinery concerning changes in the functioning of the spinal neurons and motor subsystems.
2. Supraspinal and suprasegmental mechanisms.
Spinal Mechanisms
Before discussing spinal mechanisms of spasticity. "Motor command organisation" and "Motor functions of the spinal cord", are summarized below.
Motor Control Arrangement
This system has the following components
ane. Cerebral cortex as a whole is essential for sending analytical and command motor signals for execution through:
a. Frontal motor surface area forming corticospinal (pyramidal) pathways.
b. Premotor and supplementary motor cortices which are of import for programming, i.e., sequencing and modulation of all voluntary movements.
c. Prefrontal cortex projecting to premotor and supplementary motor areas and help past planning and initiation of willed activeness.
d. Parietal cortical areas (v,7) which are important for guidance of move.
due east. Association areas acting through conscious (visual, tactile, auditory) or unconscious (proprioceptive) informations as well guide motor system.
2. Subcortical centers – basal ganglia (striatum, pallidum, substantial nigra, subthalamic nucleus) and cerebellum are important for maintenance of tone, posture, and co-ordination of movement.
3. Brainstem is the major relay station which is agile through its nuclei specially pons and medullary reticular nuclei, vestibular, and red nuclei on muscle stretch reflexes, posture, reflex, and repetitive movements.
iv. Spinal cord – contains terminal common pathways for motor execution and active through its specialized neuronal circuits and motor subsystems. Information technology involves:
a. Motor unit – consisting of a motor neuron and all the muscles it innervates, which is the functional module of motor control system. Alpha motoneurons are the final mutual pathway for the skeletal musculus activity.
b. Spinal string reflexes – raise the power of motor control arrangement for coordinated motor activity.
These include:
i. Cutaneous reflex – similar withdrawal (flexor pain nociceptive) reflex.
ii. Muscle reflex – stretch reflex.
Motor Functions of the Spinal Cord
Motor functions are basically dependent on the post-obit factors:
1. Musculus receptors and musculus stretch reflexes:
Muscle function is dependent on excitation of inductive horn motoneurons and continuous sensory feedback from each musculus to the spinal cord regarding its length and tension. Muscle spindles consisting of specialized intrafusal muscle fibers act as receptors to send information of muscle length or rate of change of length. Golgi tendon organs transmit data most tendon tension or charge per unit of change of tension. Two types of sensory endings are institute in receptor area of muscle spindle – chief (grouping Ia afferent fiber) and secondary (grouping Ii afferent fiber) (Figure 1) Golgi tendon organs send information through group Ib afferent fibers. Large alpha efferent fibers innervate extrafusal skeletal muscle fibers and small gamma efferent fibers innervate intrafusal (spindle) fibers.
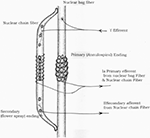
Figure 1. Diagrammatic representation of muscle spindle.
Muscle stretch reflex (myotatic reflex) is the office of the muscle spindle. Whenever a muscle is stretched, the excited spindles cause reflex contraction of the same muscle and also the synergistic muscles. "Dynamic stretch reflex" is caused by rapid stretch of the muscle and elicited through potent stimulation primarily past la afferent fibers from nuclear bag in spindle through monosynaptic pathway. Dynamic response is over within fraction of a 2nd when a weaker static stretch reflex continues for a prolonged menstruation. Static reflex is mediated by nuclear chain fires (mainly group Ii afferent and also some group la afferent fibers) acting through interneurons in the string, i.e., polysynaptically.
Muscle tone is generated by musculus spindles by acting through the stretch reflex. Muscle tone is the abiding muscular action that is necessary every bit a background to actual movement in lodge to maintain the basic attitude of the trunk peculiarly confronting the strength of gravity (Carpenter, 1984). As tone opposes movement and tends to keep muscles at preset lengths, it has to exist inverse in steps during a movement. Gamma fibers are ideally suited for this and whenever a control is sent to alpha motor fibers, gamma fibers are also excited. There occurs alpha–gamma co-activation to produce wrinkle of both extrafusal and intrafusal fibers according to the position and force commands from the brain to the spinal cord.
Clinical elicitation of stretch reflex is done in ii ways :
a. Static – by passive stretching (tone testing).
b. Dynamic – by muscle and tendon jerks.
Clonus occurs when the dynamic stretch reflex is highly sensitized and facilitated. The dynamic response dies out within a fraction of a 2d to arm-twist a new bicycle and in this fashion the muscle contraction (due east.one thousand., gastrocnemius) oscillates for a long period to produce clonus.
2. Interneurons: About integrative functions in the spinal cord are mediated by interneurons. Interneurons which are involved in every segmental and stretch reflex pathways are excited or inhibited past several peripheral and descending fiber systems (Lundberg, 1979).
Interneuron systems involved in the stretch reflex arc and in the pathophysiology of spasticity are discussed below.
1. Renshaw cells and recurrent inhibition:
Renshaw cells are situated in lamina Vii of ventral horn medial to motoneurons. Collateral from an alpha motoneuron axon excites Renshaw cell which in turn inhibits the same and also other motoneuron innervating the synergistic muscles. This alpha motoneuron-Renshaw cell – alpha motoneuron pathway of inhibition forms a negative feedback circuit to command motoneuron excitation and is called recurrent inhibition (Pompetano, 1984). In addition, Renshaw cells inhibit gamma motoneurons and la inhibitory interneurons (Jankowska and Roberts, 1972).
ii. Reciprocal la inhibition:
Stretch of a muscle activates la afferent fires to produce monosynaptic excitation of homonymous alpha motoneurons. There occurs in improver disynaptic inhibition of blastoff motoneurons innervating antagonist muscles (reciprocal inhibition). It is now established that la interneurons receive the same diverse excitatory and inhibitory inputs from segmental afferents (e.one thousand., flexor afferents) and supraspinal descending tracts as are received by alpha motoneurons (Hultborn et al., 1976). These inputs excite alpha motoneurons to contract synergistic muscles and too excite la inhibitory interneurons to inhibit in turn alpha motoneurons to combative muscles during stretch reflex activity.
Clinical electrophysiological studies by H – reflex demonstrating this miracle had been performed early past Misra and Pandey (1994) in Neurolathyrism – a pure motor spastic tropical paraparesis acquired by ingestion of grass peas containing the neurotoxin beta – ODAP. These authors demonstrated increased motoneuron excitability, altered transmission in the premotoneuronal pathway and lack of reciprocal inhibition in the generation of spasticity in this condition. More than recently, Crone et al. (2007) likewise demonstrated reduced reciprocal inhibition in neurolathyrism.
three. Inhibition from group II afferents:
In addition to established role of grouping II fibers in stretch reflex arc, these fibers from secondary spindle endings are known to produce flexion reflex by exciting flexor alpha motoneurons and inhibiting extensor motoneurons.
4. Non-reciprocal lb inhibition:
1b afferent fibers from Golgi tendon organs finish on lb inhibitory interneurons which synapse with alpha motoneurons to both homonymous and heteronymous muscles. Like Renshaw cell and la inhibitory interneurons, lb interneurons also receive diverse segmental and supraspinal inputs. Therefore, lb inhibition is not a simple autogenic inhibitory condom mechanism to regulate muscle tension only. It is a office of complex system regulating musculus tension to control posture and movement.
5. Presynaptic inhibition:
The amplitude of EPSP generated in a motoneuron in response to la afferent stimulation diminishes if in that location occurs prior depolarization of this la afferent fiber through axo–axonic synapse with a specific interneuron. The specific interneurons involved in this process of presynaptic inhibition are also controlled by descending pathways. This permits automatic suppression of unimportant afferent informations (Schmidt, 1971).
vi. Flexor reflex afferents
Nociceptive reflex or simply pain reflex produces contraction of flexor muscles of a limb (withdrawal) and crossed extensor reflex of opposite limb. This is mediated by polysynaptic connexion betwixt flexor reflex afferents (FRA), interneurons and motoneurons of extensor as well as flexor muscles.
Office of Spinal Excitatory Mechanisms in Spasticity
1. Increased fusimotor drive: Exaggerated musculus stretch reflexes in spasticity was attributed to the increased sensitivity of the muscle spindles due to increased fusimotor activity some 20–xxx years ago. The posterior root department for handling of spasticity in cerebral palsy and diluted procaine injection well-nigh intramuscular nerve for treating hyperactive stretch reflex (Rushworth, 1960) were based on this theory. The local anesthetic injection was assumed to block pocket-sized diameter fusimotor fibers but not larger diameter alpha motor axons. Later on experiments using microneurography studies (Hagbarth, 1981) failed to demonstrate any change in the belch of muscle spindle afferents in spastic patients making it unlikely that any pregnant changes in fusimotor drive exist. The hypothesis that increased fusimotor outflow is involved in the pathophysiology of spasticity has consequently been discredited.
2. Primary hyperexcitability of blastoff motoneurons following spinal lesions – plateau potentials: Recent research has shown that several active membrane properties tin shape the motoneuronal output (Rekling et al., 2000; Powers and Binder, 2001; Heckman et al., 2003; Hultborn et al., 2004; Heckmann et al., 2005). Voltage dependent, persistent inward Caii+ and Na+ currents are of item relevance, every bit they amplify and prolong the response of motoneurons to synaptic excitation. These inward currents can produce prolonged depolarizations (plateau potentials) when opposing outward currents are reduced or the Ca2+ channels are facilitated, e.g., by serotonergic and noradrenergic innervations of the motoneurons.
When a graded depolarizing electric current is introduced through an intracellular electrode into a motoneuron of a decerebrate true cat, a critical threshold (plateau threshold) is reached. Higher up this threshold, further depolarization will trigger a regenerative activation of sustained in current. In the decerebrate cat (with tonic descending serotonergic drive) the plateau potentials are easily evoked. However, following an acute spinal transaction they cannot be evoked unless the persistent inward electric current is specifically increased, e.yard., by monoaminergic agonist. In a few cases, it was possible to demonstrate that plateau potentials tin once again be induced in the chronic spinal land without calculation any neurotransmitter precursors or agonists (Feganel and Dumtrijevic, 1982). This suggested that plateau potentials, returning long afterwards spinal injury, can play a role in the pathophysiology of spasticity. Lilliputian is known about the possible contribution of plateau potentials to the development of spasticity in humans considering of the difficulty in demonstrating the existence of such intrinsic membrane properties in the intact organism.
three. Enhanced cutaneous reflexes: In spasticity, cutaneous reflexes (flexor or withdrawal) are enhanced. Dorsal horn neurons give rise to both long axons which form ascending tracts and brusk propriospinal axons to innervate motor neurons of cord. Rostral lesions in CNS disrupting descending reticulospinal tract (RST) or spinothalamic tract alter normal gating mechanisms in dorsal horn so that pain is experienced to rather innocuous stimuli. This mishandling of segmental inputs helped by failure of presynaptic inhibition (mediated through GABA-ergic synapses on principal afferents in substantia gelatinosa) results in hyperactivity in long tract neurons to be felt every bit pain equally an associated characteristic in spasticity.
Similarly excitation of brusk propriospinal interneuron organization in the cord produces hyperactive nociceptive reflexes. This system acts as an arousal system for motoneurons in absenteeism of brainstem reticular organisation in a cord deprived of supraspinal influences (Burke and Ashby, 1972). The clinical signs of this phenomenon include Babinski's response, triple flexion of leg and gross flexor, or sometimes extensor spasm which may be produced past uncomplicated and non-noxious cutaneous stimuli.
Role of Spinal Inhibitory Mechanisms in Spasticity
Current hypotheses stress more on alterations in inhibitory mechanisms in spinal neuronal circuitry than an excitatory processes although both may be inter-related in a patient with spasticity.
Figure 2 illustrates the spinal reflex circuits that could be involved in the development of spasticity. The monosynaptic Ia excitation which underlies the dynamic and tonic components of the stretch reflex may exist inhibited by various spinal reflexes pathways.
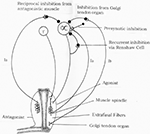
Figure 2. Spinal pathways which may exist responsible for development of spasticity.
These include:
1. Presynaptic inhibition of Ia afferent terminals.
ii. Disynaptic reciproval Ia inhibition from muscle spindle Ia afferents from the antagonist muscles.
3. Recurrent inhibition via motor axon collaterals and Renshaw cells.
4. Non-reciprocal Ib inhibition from Golgi tendon organs.
five. Inhibition from muscle spindle grouping 2 afferents (not shown in the Figure 3).
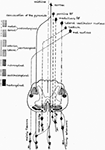
Figure 3. Supraspinal descending pathways in spinal cord (RF, reticular formation).
The changes in reflex manual in these pathways may depend both on an altered supraspinal bulldoze (if any remains) and on secondary changes at cellular level in the spinal cord below the lesion which may include:
one. Presynaptic inhibition of Ia afferent terminals:
As discussed before, the inhibition is through axo–axonic synapses which are GABA-ergic and on activation reduces the amount of transmitter released by Ia terminals on the motoneuron. If there occurs a reduction in the commonly maintained tonic level of presynaptic inhibition, in that location will be increased response on blastoff motoneurons by Ia input and spasticity may ensure.
A technique used to study presynaptic inhibition in homo subjects was to vibrate the Achilles tendon and record the resulting low of the soleus H-reflex (Shush and Ashby, 1972; Ashby et al., 1974). As this vibratory inhibition was subsequently institute to be decreased in spastic patients, information technology became generally accepted that spasticity involved reduced presynaptic inhibition of Ia afferents (Ashby et al., 1974; Ashby and Verrier, 1975, 1976). All the same, later studies have cast doubts on this estimation. Using a more optimal technique to evaluate presynaptic inhibition, Nielsen et al. (1995) demonstrated that presynaptic inhibition was reduced in spastic patients with multiple sclerosis. A similar finding was made for patients with spinal cord injury, but not for hemiplegic stroke patients (Paist et al., 1994). Presynaptic inhibition thus seems to be reduced in some spastic patients just not in all.
2. Disynaptic reciprocal Ia inhibition:
Reduced reciprocal inhibition is a potent candidate for playing a major office in the pathophysiology of spasticity (Crone and Nielsen, 1994; Crone et al., 2004, 2006). In spastic patients, reflex spread is common with reflex induced co-contraction of adversary muscle groups, a failure of reciprocal inhibition. The Ia inhibitory interneurons are activated past descending motor fibers, damage to which could reduce this type of inhibition.
three. Recurrent inhibition:
Recurrent inhibition mediated by Renshaw cells take been studied by complex H-reflex techniques (Pierrot-Deseilligny and Bussel, 1975). In some patients with both supraspinal too as traumatic spinal lesions increased recurrent inhibition may exist seen, which patently plays no role in development of spasticity (Katz and Pierrot-Deseilligny, 1982; Shefner et al., 1992). Just in patients with progressive paraparesis of ALS is a reduction establish at rest and it is doubtful that this reduction contributes to the spasticity observed in these patients (Mazzochio and Rossi, 1989; Shefner et al., 1992). Changes in recurrent inhibition thus probably plays no major role in the pathophysiology of spasticity.
4. Non-reciprocal Ib inhibition:
This inhibition is acquired past activation of Ib afferents coming from Golgi tendon organs and is mediated by segmental interneurons projecting to mononeurons of the aforementioned muscle. Ib inhibition may exist demonstrated in human subjects past specialized H reflex studies (Raynor and Shefner, 1994). Whereas this inhibition is easily demonstrated in healthy subjects, in that location was failure to produce any inhibition on the paretic side in hemiplegic patients, along with a facilitatory effect in some subjects (Pierrot-Deseilligny et al., 1979). This observation suggests that alteration of Ib inhibition excitation plays a role in the pathophysiology of spasticity. All the same, reflex effects from Golgi tendon Ib afferents are unchanged later on spinal string lesions in humans.
At this stage it is important to take annotation of the fact that the anterior horn cell (AHC) or spinal motoneuron is the central nucleus in the operation of all spinal reflexes. Dysfunction of AHC leads to hypotonicity – every bit is evident in pure AHC affecting diseases like poliomyelitis, spinal muscular atrophy and the progressive muscular atrophic grade of motor neuron illness (MND). Associated dysfunction of supraspinal pathways with some surviving spinal motoneurons might cause spastic weakness as commonly seen in the amyotrophic lateral sclerosis form of MND.
Supraspinal and Suprasegmental Mechanisms
The importance of supraspinal and suprasegmental control of spinal reflexes was progressively understood since the role of muscle stretch reflex to generate musculus wrinkle was discovered by Liddell and Sherrington (1924), Delwaide and Oliver (1988) Descending influences control spinal reflexes by converging forth with chief peripheral afferents on common interneuronal pool projecting to motoneurons. Imbalance of the descending inhibitory and facilitatory influences on muscle stretch reflexes is thought to exist the crusade of spasticity (Lundberg, 1975). These influences are discussed below.
There are 5 important descending tracts, of these, corticospinal tract originates from cerebral cortex. Other 4 come from closely neighboring parts in the brain stalk and these are – Reticulospinal Vestibulospinal, Rubrospinal, and Tectospinal tracts. In homo spastic paretic syndrome, the three important pathways are – corticospinal, reticulospinal, and vestibulospinal.
Inhibitory Supraspinal Pathways
1. Corticospinal pathway – Isolated pyramidal lesions take non produced spasticity in conditions such every bit destruction of motor cortex (area 4), unilateral lesion in cerebral peduncle, lesions in footing pontis and medullary pyramid (Bucy et al., 1964; Brooks, 1986). Instead of spasticity these lesions produced weakness, hypotonia, and hyporeflexia. Pyramidal tract lesion alone is more responsible for weakness and loss of superficial reflexes such every bit abdominal reflexes rather than spasticity, hyper-reflexia and Babinski's sign. Spasticity however may be caused in lesions of area 4 if the lesions include the premotor and supplementary motor areas. Fibers responsible for spasticity run with the pyramidal tract to end in the bulbar reticular formation (corticoreticular pathway). Lesions (vascular) in the anterior limb of internal sheathing and not in the posterior limb produce spasticity equally fibers from supplementary motor area pass through anterior limb. Large eye cognitive artery territory infarcts involving corticospinal and corticoreticular pathways produce spasticity (Gilman et al., 1973). Failure of isolated pyramidal lesion to produce spasticity does not however infer that this tract has no influence over musculus tone. Ipsilateral supplementary motor and premotor areas and contralateral motor cortex can take up some of the functions of pyramidal tract and foreclose spasticity to develop.
2. Corticoreticular pathways and dorsal reticulospinal tract
Medullary reticular formation is active as a powerful inhibitory center to regulate muscle tone (stretch reflex) and the cortical motor areas control tone through this heart. Lesions of premotor area (frontal cortex) or internal capsule reduces control over medullary center to produce hypertonicity.
Dorsal RST situated in the ventral part of the lateral funiculus of the spinal cord carries the inhibitory influence from the medullary heart. This tract is non-monoaminergic, but dissimilar ventral (medial) RST, information technology inhibits FRA besides as stretch reflex arc. "Flexor spams" are release phenomenon of flexor reflexes due to damage to dorsal reticulospinal pathway (Fisher and Curry, 1965). Clasp-knife phenomenon is also a release miracle due to loss of inhibitory effects on FRA.
Excitatory Supraspinal Pathways
1. Vestibulospinal pathway: Vestibulospinal tract (VST) is a descending motor tract originating from lateral vestibular (Deiter's) nucleus and is almost uncrossed. The tract ends mostly on interneurons only besides excites motor neurons monosynaptically. This excitatory pathway helps to maintain posture and to support against gravity and so control extensors rather than flexors. This pathway is of import in maintaining decerebrate rigidity merely has bottom role in homo spasticity (Fries et al., 1993).
The cerebellum through its connections with the vestibular nuclei and reticular germination may indirectly modulate musculus stretch reflexes and tone.
2. Medial (ventral) RST – Through this tract reticular formation exerts facilitatory influence on spasticity. The tract has a diffuse origin being mainly from pontine tegmentum. Dissimilar dorsal RST, it is not affected past stimulation of motor cortex or internal capsule and not inhibitory to FRA. This pathway is more important than vestibulospinal organization in maintaining spastic extensor tone (Schreiner et al., 1949; Shahani and Young, 1973).
Clinical Correlations in Lesions of Descending Pathways
The four descending pathways which are important in spastic paretic syndrome are arranged as follows in the spinal cord:
one. Lateral funiculus contains corticospinal tract (CST) and dorsal RST.
2. Inductive funiculus contains VST and medical RST (in close proximity with medial longitudinal fasciculus).
Muscle tone is maintained past a controlled balance on stretch reflex arc by inhibitory influence of CST and dorsal RST and facilitatory influence (on extensor tone) by medial RST and to a lesser extent in humans by VST.
i. In cortical and internal capsular lesions, the decision-making drive on the inhibitory centre in the medullary brain stem is lost and so in absenteeism of inhibitory influence of dorsal RST originating from this center, facilitatory activity of medial RST becomes unopposed. This results in spastic hemiplegia with antigravity posturing, but flexor spams are unusual.
ii. Spinal lesions – (a) Incomplete (partial) myelopathy involving lateral funiculus (e.g., early multiple sclerosis) (Peterson et al., 1975) may bear on CST only to produce paresis, hypotonia, hyporeflexia, and loss of cutaneous reflexes. If dorsal RST is involved in addition, unopposed medial RST action then results in hyper-reflexia and spasticity (like to cortical or capsular lesions), the latter being marked in antigravity muscles to produce paraplegia in extension. Extensor and flexor spasms may occur, the former being commoner.
(b) Severe myelopathy with involvement of all the 4 descending pathways produces less marked spasticity compared to isolated lateral cord lesion because of lack of unopposed excitatory influences of medial RST and VST. The latter cistron is also responsible for lack of extensor hypertonia and in presence of release of flexor reflexes by dorsal RST lesion, helps to produce paraplegia in flexion. Paraplegia in flexion is also possible in partial myelopathy if FRA go stimulated past factors like pressure sores.
(c) Isolated dorsal RST involvement with CST sparing (proved pathologically and electrophysiologically) (Oppenheimer, 1978; Thompson et al., 1987) may explicate marked spasticity and spasms just little weakness in many cases of spastic paraparesis. But hyper-reflexia with normal tone is again a possibility in isolated inductive cord lesion.
3. Clinically spasticity may be of different types due to involvement of descending pathways. Depending on the predominant involvement of phasic (dynamic) or tonic (static) components of musculus stretch reflexes, the spasticity may be "phasic" and "tonic" Precollicular lesions in cat produce substantially phasic and decerebration at a lower level produces essentially tonic spasticity (Shush et al., 1972). Patients of chronic spinal cord injury who are ambulatory with minimum voluntary movement reveals more of "phasic" spasticity in the grade of increased tendon jerks and clonus. Non-ambulatory patients with or without voluntary movement revels more of "tonic" spasticity on passive stretch at ankle and vibratory tonic reflex testing by noting tonic response of triceps surae on vibrating the Achilles tendon (Burke et al., 1972).
4. Neuroplasticity of the spinal string in the form of receptor supersensitivity of neurons to a loss of synaptic input and sprouting of axon terminals are also responsible for hypertonicity in complete myelopathy with delayed reorganization after a variable period of spinal daze (Davis, 2000). This hypertonicity is not velocity dependent as in partial myelopathy and results from nearly continuous flexor spasms. Paraplegia in flexion may be associated with mass reflexes (exaggerated flexor spasms) in this condition.
five. Cerebellum and muscle tone: The cerebellum does not seem to have a direct effect on musculus tone determining spinal reflex pathways as there is no direct descending cerebello-spinal tract. The ascending spino-cerebellar tracts bear afferent impulses relating to joint and limb position and range and management of movement. Such impulses are also carried past the posterior column tracts to the medullary centers and then onto the cerebellar cortex through the inferior cerebellar peduncles. The cognitive and cerebellar cortices are inter-connected by feed-back–feed-forward loops which projection through the corticospinal and other descending extrapyramidal pathways to the spinal string. The cerebellum mainly influences musculus tone through its connections with the vestibular and brain stem reticular nuclei. Pure cerebellar lesions classically produce hypotonia. But associated corticospinal tract involvement produces varying degrees of spasticity equally seen in some forms of spino-cerebellar atrophies (SCA) and the spastic ataxia of Charlevoix–Saguenay encountered in French Canadian stock. On the other hand chronic cerebellar stimulation had been used to relieve spasticity in cerebral palsy (Ebner et al., 1982). Earlier experimental study demonstrated that cerebellar surface stimulation reduced the aamplitude of the tonic and phasic stretch reflexes (Dimitrijevic, 1984). This modified the organisation of the segmental reflexes producing a more normal reciprocal relationships of EMG activity in the agonist and antagonist.
Overview of Mechanisms
How does UMN lesion crusade spasticity and associated phenomena? The major problem is a loss of control of the spinal reflexes. Spinal reflex activity is normally tightly regulated and if inhibitory command is lost, the balance is tipped in favor of excitation, resulting in hyperexcitability of the spinal reflexes. The problem is made difficult by the fact that individual patients take lesions affecting dissimilar pathways to dissimilar extent and that the subsequent adaptations in the spinal networks, as a issue to the principal lesion, may vary considerably. The dissimilar spinal mechanisms – plateau potentials, reciprocal inhibition and presynaptic inhibition – may take different roles in unlike patients. Information technology is likely that spasticity is non acquired past a single mechanism, but rather by an intricate concatenation of alterations in dissimilar inter-dependent networks.
The fact that there is a period of stupor, followed past a transition menstruation when reflexes return, only are non hyperactive suggests that this is not just simply a question of switching off supraspinal inhibition, or altering the balance. It implies that in that location must be some sort of rearrangement, a kind of neuronal plasticity, occurring inside the spinal cord, and most probably at the cerebral level as well. Ane possibility is sprouting of afferent axons (Raisman, 1969; Benecke, 1985; Raineteau and Schwab, 2001; Bareyre et al., 2004). Afferent fibers might sprout, adhere to previously inhibitory synapses, and catechumen them to excitatory synapses. Alternatively there could be development of denervation hypersensitivity due to upregulation of receptors (Sravraky, 1961).
Spasticity may also be explained by changes in mechanical properties of muscles and not only by hyper-reflexia (Dietz et al., 1981; Thilmann et al., 1991). The increased mechanical resistance may be caused past alterations in tendon compliance and physiological changes in muscle fibers which bear on functional motility of leg occurring at low athwart velocities. Contractures are farthermost furnishings of mechanical resistance which can be prevented by early treatment of hypertonia with botulinum toxin (BTX) in spastic cognitive palsy.
In decision, information technology needs to be mentioned that the progress being fabricated in the electrophysiologic assay of spinal control mechanisms in spasticity and measurement of spasticity are helpful for greater agreement of pathophysiology of the condition. Newly used drugs have multiple sites of actions (Delwarde and Pennisi, 1994). On the whole information technology seems highly likely that more i pathophysiologic abnormality contributes to development of spasticity (Sheean, 2001; Nielsen et al., 2007).
Mechanisms of Deportment of Anti-Spasticity Drugs – A Brief Note
The apply of BTX in treatment of spasticity has already been mentioned. When injected at or about the motor bespeak of affected muscle BTX binds to the SV2 receptor on the presynaptic membrane assuasive for entry of the toxin into the axon final. Once inside the axon, BTX light chains human action to impede exocytosis of acetylcholine (ACH). This allows for fusion of neurotransmitter-containing intra-axonal vesicles with the presynaptic membrane, resulting in extrusion of ACH into the synaptic cleft. The reduced presynaptic outflow of ACH at the neuromuscular junction causes diminution of musculus contraction. BTX reduces the frequency and quantity merely non the amplitude of miniature endplate potential (MEPP). The motor EPP is reduced below the muscle membrane threshold and the ability to generate musculus cobweb activity potentials and subsequent contraction is diminished (Ney and Joseph, 2007).
Of the orally active agents, Baclofen is a centrally acting GABA analog. It binds to GABA receptor at the presynaptic terminal and inhibits musculus stretch reflex. Baclofen can also be used intrathecally.
Dantrolene interferes with the release of calcium from the sarcoplasmic reticulum of the muscle.
Tizanidine is an imidazole derivative with agonist action on blastoff-two-adrenergic receptors in the central nervous arrangement. The verbal mechanism of its action in reduction of homo musculus tone is not known but a central action tin be speculated.
Conflict of Interest Statement
The authors declare that the enquiry was conducted in the absenteeism of whatever commercial or financial relationships that could exist construed as a potential conflict of interest.
References
Ashby, P., and Verrier, One thousand. (1975). Neurophysiological changes post-obit spinal cord lesions in man. Tin. J. Neurol. Sci. 2, 91–100.
Pubmed Abstract | Pubmed Full Text
Ashby, P., and Verrier, Grand. (1976). Neurophysiologic changes in hemiplegia. Possible caption for the initial disparity between muscle tone and tendon reflexes. Neurology 26, 1145–1151.
Pubmed Abstract | Pubmed Full Text
Bareyre, F. Chiliad., Kerschensteiner, M., Rainetear, O., Mettenleiter, T. C., Weinmann, O., and Schwab, M. E. (2004). The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Benecke, R. (1985). "Basic neurophysiological mechanisms in spasticity," in Treating Spasticity. Pharmacological Advances, ed. C. D. Marsden (London: Hans Huber Publishers), 11–17.
Brooks, V. B. (1986). The Neural Basis of Motor Control. New York: Oxford University Press.
Bucy, P. C., Kephnger, J. E., and Siqucira, E. B. (1964). Destruction of the pyramidal tract in man. J. Neurosurg. 21, 385–398.
CrossRef Full Text
Carpenter, R. H. Due south. (1984). Neurophysiology. London: Arnold Heinmann, p. 234.
Crone, C., Johnsen, L. L., Bieringsorensen, F., and Nielsen, J. B. (2006). Advent of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126, 495–507.
CrossRef Full Text
Crone, C., Petersen, Northward. T., Gimenz-Roldan, S., Lungholt, B., Nyborg, K., and Neilsen, J. B. (2007). Reduced reciprocal inhibition is seen merely in spastic limbs in patients with neurolathyrism. Exp. Brain Res. 181, 193–197.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Crone, C., Petersen, N. T., Nielsen, J. Eastward., Hansen, N. Fifty., and Nielsen, J. B. (2004). Reciprocal inhibition and corticospinal transmission in the arm and leg in patients with autosomal ascendant pure spastic paraparesis (ADPSP). Brain 127, 2693–2702.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Delwaide, P. J., and Gerard, P. (1993). "Reduction of not-reciprocal (lb) inhibition: a key factor for interpreting spastic musculus stiffness," International Congress on Stroke Rehabilitation, Berlin.
Delwarde, P. J., and Pennisi, G. (1994). Tizanidine and electrophysiologic analysis of spinal control mechanisms in human with spasticity. Neurology 44 (11 Suppl. 9), S21–S28.
Pubmed Abstract | Pubmed Total Text
Dietz, V., Quimern, J., and Berger, W. (1981). Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 104, 431–449.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Dimitrijevic, M. R. (1984). "Neural control of chronic upper motor neuron syndromes," in Electromyography in CNS Disorders, ed B. T. Shahani (Boston, London: Butterworths), 111–127.
Gilman, S., Marco, L. A., and Ebel, H. C. (1973). Furnishings of medullary pyramidotomy in the monkey. II. Abnormalities of spindle afferent response. Encephalon 94, 515–530.
CrossRef Full Text
Hagbarth, K. E. (1981). "Fusimotor and stretch reflex functions studied in recordings from muscle spindle afferents in man," in Muscle Receptors and Move, Vol. xiii. eds A. Taylor and A. Prochazka (New York: Oxford Academy Press), 109–115.
Hultborn, H., Brownstone, R. B., Toth, T. I., and Gossard, J. P. (2004). Key mechanisms for setting the input-output gain across the motoneuron pool. Prog. Brain Res. 143, 77–95.
Pubmed Abstract | Pubmed Full Text
Hultborn, H., Illert, Thou., and Santini, M. (1976). Convergence on interneurones mediating the reciprocal la inhibition of motoneurons. III. Effects from supraspinal pathways. Acta Physiol. Scand. 96, 368–391.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Jankowska, East., and Roberts, Westward. J. (1972). Synaptic actions of unmarried interneurons mediating reciprocal la inhibition of motoneurons. J. Physiol. (Lond) 222, 623–642.
Pubmed Abstruse | Pubmed Full Text
Lance, J. Due west. (1980). "Symposium," in Spasticity: Disordered Motor Control, eds R. 1000. Feldman, R. R. Immature, and W. P. Koella (Chicago: Year Volume Medical Pubs), 485–495.
Liddell, Due east. G. T., and Sherrington, C. S. (1924). Reflexes in response to stretch (myotatic reflexes). Proc. R. Soc. 96B, 212–242.
Lundberg, A. (1975). "Control of spinal machinery from the brain," in The Nervous System, Vol. 1, eds D. Tower and R. Brady (New York: Raven Printing), 253–265.
Mazzochio, R., and Rossi, A. (1989). Recurrent inhibition in human spinal spasticity. Int. J. Neurol. Sci. 10, 337–347.
CrossRef Full Text
Ney, J. P., and Joseph, K. R. (2007). Neurologic uses of Botulinum toxin blazon A. Neuropsychiatr. Dis. Care for. 6, 785–790.
Nielsen, J. B., Crone, C., and Hultborn, H. (2007). The spinal pathophysiology of spasticity – from a basic science point of view. Acta Physiol. 189, 171–180.
CrossRef Total Text
Paist, Thou., Mazevet, D., Dietz, Five., and Pierrot-Descilligny, E. (1994). A quantitative assessment of presynaptic inhibition of la afferents in spastics. Differences in hemiplegics and paraplegics. Encephalon 117, 1449–1455.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Pierrot-Deseilligny, Eastward., and Bussel, B. (1975). Evidence for recurrent inhibition by motoneuron in man subjects. Brain 88, 105–108.
CrossRef Full Text
Pompetano, O. (1984). "Recurrent inhibition," in Handbook of the Spinal Cord, Vols. 2 and iii, ed. F. A. Davidoff (New York: Marcel Dekker), 461–557.
Raynor, E. Yard., and Shefner, J. Thou. (1994). Recurrent inhibition is decreased in patients with amyotrophic lateral sclerosis. Neurology 44, 2148–2150.
Pubmed Abstract | Pubmed Full Text
Rekling, J. C., Funk, G. D., Bayliss, D. A., Dong, X. W., and Feldman, J. L. (2000). Sunaptic control of motoneuronal excitability Phys. Rev. 80, 767–852.
Schmidt, R. F. (1971). Presynaptic inhibition in the vertebrate central nervous system. Ergeb. Physiol. 63, 91–101.
Schreiner, L. M., Mandsley, D. B., and Magoum, H. West. (1949). Role of encephalon stem facilitatory systems in maintenance of spasticity. Neurophysiology 12, 207–216.
Pubmed Abstract | Pubmed Full Text
Shahani, B. T., and Young, R. R. (1973). "Human flexor spasm," in New Developments in Electromyography and Clinical Neurophysiology, Vol. 3, ed J. E. Desmedt (Basal: South Karger AG), 734–743.
Sheean, Thou. (2001). The pathophysiology of spasticity. Eur. J. Neurol. 9 (Suppl. i), iii–ix.
CrossRef Full Text
Shefner, J. M., Berman, Southward. A., Sarkarati, 1000., and Immature, R. R. (1992). Recurrent inhibition is increased in patients with spinal string injury. Neurology 42, 2162–2168.
Pubmed Abstruse | Pubmed Total Text
Sravraky, 1000. W. (1961). Supersensitivity Following Lesions of the Nervous Arrangement. An Attribute of the Relativity of Nervous Integration. Toronto: University of Toronto Press, pp. 1–210.
Thilmann, A. F., Fellows, Due south. J., and Grams, Eastward. (1991). The mechanism of spastic muscle hypertonus: variation in reflex proceeds over the fourth dimension course of spasticity. Brain 114, 233–244.
Pubmed Abstract | Pubmed Full Text
Thompson, P. D., Twenty-four hour period, B. L., and Rothwell, J. C. (1987). The estimation of electromyographic responses to electric stimulation of the motor cortex in diseases of the upper motor neuron. J. Neurol. Sci. fourscore, 91–110.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Source: https://www.frontiersin.org/articles/10.3389/fneur.2010.00149/full
0 Response to "Review Muscle Tone and Abnormalities in Muscle Tone by Completing Each Sentence Below"
Post a Comment